Smart Water Quality Monitoring System Using IoT
Written by R. Pamila, Ajith Kumar M, Ramesh Aravinth R, and Sanjay V.
Water is essential to human life and health. Water and irrigation play crucial roles in industrial and commercial purposes. In recent years, sudden population increases have caused water scarcity, which poses a health hazard. The need for water is incredibly high, which leads to more fresh water extraction. Population growth also increases environmental pollution, which contributes to the pollution of water quality. This places us in the position to filter and monitor water quality using smart technologies like IoT. Due to its charcoal content, we intend to use organic waste as a biochar in place of a charcoal layer in the filtering media that treat water. To monitor the quality of treated water, we are using IoT techniques such as raspberry pi, PH sensors, Turbidity sensors, and ultrasonic sensors to determine the outputs.
A monitoring system based on a wireless sensor network and IoT is essential in order to establish a viable quality of water. This system should include an intelligent electronic monitoring technique in which the application displays the parameters such as pH value, turbidity and conductivity level. These water datapoints would then be sent to the IOT server. With the help of a wireless GSM (Global System for Mobile communication), the customer would be informed about the quality of water. Within this system, water passes through a biochar filter before being tested and analyzed.
Chapter 1
Introduction
Water is essential to human life and health. It is nature’s gift to humankind. Water and irrigation play crucial roles in industrial and commercial purposes. It is also important to the development of any city of the world. In recent years, sudden population increases have caused water scarcity, which poses a health hazard. 80% of consumed water is turned to waste that should be treated and reused. The need for water is incredibly high, which leads to more fresh water extraction. Water is a non-renewable resource that becomes more scarce day by day as a result of manmade activities.
Population growth increases environmental pollution, which contributes to the pollution of water quality. This places us in the position to filter and monitor water quality using smart technologies like IoT.
Due to its charcoal content, we intend to use organic waste as a biochar in place of a charcoal layer in the filtering media that treat water. Biochar is a type of charcoal layer made of organic and agricultural waste. The major crop residues produced in India are paddy and wheat. In India, most agricultural waste is burned, which causes air pollution and contributes to global warming. In this alternative, agricultural waste is used as a filtration media to treat waste water and reuse it for the user’s purposes. Water passes through a biochar filter, and then exits from the filter media with fewer impurities. Rather than burn agricultural waste, we can use it as a reusable material. This method can help reduce fresh water extraction, water scarcity, and waste generation.
1.1 Objective
With reduced freshwater extraction from rivers and aquifers, there is the less environmental impact from septic tanks and water treatment plants. There is also reduced energy use and chemical pollution. When there is less freshwater extraction, groundwater is recharged as it reclaims nutrients. Among our objectives are meeting the increasing demand of water and creating awareness around conserving water.
The use of gray water at decentralized sites for landscape irrigation and toilet flushing reduces the amount of potable water distributed to these sites, along with the amount of fertilizer needed, and the amount of wastewater generated, transported and treated at wastewater treatment facilities. In other words, water reuse saves water, energy, and money. By implementing IoT, we can check the quality of water very easily while actually saving time.
1.2 Scope
- This water would be used for landscape irrigation and toilet flushing.
- Recycled water can be spread or injected into ground water aquifers to augment ground water supplies.
- Grey water enables a landscape to flourish where water may not otherwise be available to support much plant growth.
1.3 Literature Review
Prof. Abeer Albalawneh conducted research in 2015 in his Review of the Grey Water and proposed a Grey Water recycling scheme for agricultural, irrigation reuses. As per his research, Grey Water treatment methods vary based on site conditions and Grey Water characteristics. Water consumption always depends on quality-of-life standards and the availability of resources. The ranges of electrical conductivity, turbidity, and suspended solids for dark Grey Water are 190- 1,830uS cm-1, 19-444 NTU and12-315 mgL-1, respectively, while for light Grey Water, these ranges are 14-921 µScm- 1.12.6-375 NTU, and 29-505 mgL-1, respectively. Physical Grey Water treatment systems include filtration and sedimentation. A mean removal of 30% COD and a maximum E. coli removal of two log CFU/100 ml was observed. Electro coagulation was effective at treating shower water from a building.
Prof. R. T. Pachkor conducted research in 2017 on the Integrated approach for grey water treatment. As per his research, grey water comprises 50-80% of residential waste water. Grey water gets its name from its cloudy appearance and from its status as neither fresh nor heavily polluted. Grey water is a domestic site's waste water that is collected from dwelling units, commercial buildings, and institutions of the community. Many new or modified treatment processes being used are becoming a necessary facet of good management. In this method, the quantification of greywater was determined by direct methods like water meters and the bucket method, as well as indirect methods, like water consumption and types of uses.
Prof. J. S. Lambe conducted research on grey water treatment and reuse. As per his research in a geographical area that forms 2.4% of the world's land area, water used in bathing and hand washing purposes produces around 50- 60% of total Grey Water, and it is assumed to be the least contaminated type of Grey Water. It readily promotes and supports the growth of micro-organisms. Grey Water from kitchens generates about 10% of all Grey Water. Grey Water reuse methods can range from low-cost methods, such as the manual bucketing of Grey Water from the outlet of bathrooms, to primary treatment methods that coarsely screen oils.
Rawa Al-Jarallah conducted research in 2013 on the potential for reusing Grey Water and its generation rates for sustainable potable security in Kuwait. As per research, the amount of fresh water produced will not be sufficient to meet the demand. Indeed, the shortage of fresh water is becoming a global problem. This issue has caused the scientific community to explore alternative water sources, such as wastewater reuse. The Grey Water generated as a percentage of the total water consumption differs from one country to the next based on age, gender, living standards, habits, and the degree of water abundance. In Australia, 50% of the water consumed is transformed into Grey Water and reused in toilet flushing and irrigation, reducing the total water consumption by 29%. There are untreated Grey Water treatment systems and biological treatment systems (Environmental Agency, 2011). The selection of a treatment system is based on the ultimate use.
Jie Zhu conducted research in 2018 on the feasibility of on-site Grey Water reuse for toilet flushing in China. As per his research, drought, and water shortage are the key factors for water scarcity in arid and semi-arid areas. As a result, there is a growing need to manage water resources in a sustainable manner. As for water reuse, current technology manages to treat waste water into any desired quality. Compared to household composite domestic wastewater, Grey Water is more lightly polluted, thus the treatment process should be optimized. The key problem is how to balance the investment and treatment costs with reuse criteria. In this paper, a summary of the current Grey Water treatment systems is given, and advice on its application is provided. Recently, the most commonly described and promising application for Grey Water reuse has been toilet/urinal flushing, which can reduce water demand within a dwelling by up to 10-30%.
Narges Shamabadi et al., (2015) recommends Grey Water treatment systems that use trickling filters composed with a suspended plastic medium. The method suggests a mechanism in which the waste matter (if existent) gets removed by a mesh composite of 1cm thickness. Then, the Grey Water gets collected in a buried tank and the outlet water is pushed through a pump to the trickling filter. The water gets back to the tank counting thrice the process. The water from the trickling filter is led to the settling tank, where the sludge produced as an end result gets settled down. Finally, the water is passed to chlorination system to be sterilized.
K.A.Vakil et al., (2014) presents a new concept of Grey Water treatment through an electrochemical reactor that runs with a supply voltage of 12V Dc. Erwin Nolde (1999) describes the reuse of greywater in a multi-storey building with natural treatment using sand filters with reed beds and biological treatments using membrane filtration with a UV disinfection setup. The paper provides choices in selecting a filtration process based on economical perspective.
Moslemi Zadeh et al., (2013), in their paper, suggest that Grey Water (GW) recycling helps urban water management system to use it for non-portable activities like urinal and toilet flushing. This strategy helps to alleviate the risks associated with water demand. The paper initiates a novel method of a cross connection system that accumulates Grey Water from residential buildings and recycles it for flushing urinal/toilet in both residential and commercial buildings.
Chapter 2 - Introduction and Test for Materials Used
2.1 Biochar
A carbon-enriched biomaterial known as biochar is produced during the pyrolysis process, which involves burning biomass. A pyrogenic carbonaceous material is biochar (PCM). It is a general word for any substances made by thermochemical conversion and containing organic carbon. Biomass pyrolysis, which breaks down organic materials at temperatures between 350 and 1000 °C, produce biochar (ebc guideline). These produced materials include biochar, charcoal, soot, and activated carbon. Biomass is thermochemically converted into charcoal, which is primarily used for the production of energy. The term "activated carbon" is used when charcoal has undergone additional processing and has been made active by adding chemicals or steam. Biochar is the end result of the thermochemical conversion of biomass in a low-oxygen environment. The most common and essential component of concrete is cement.
Figure 2.1: Biochar.
2.1.1 Banana Peel As Biochar
Scientists have discovered that banana peels, which are known for causing slips, may also prevent contaminants from slipping into your water. Peels from bananas can be used as a water filter. Sulfur, nitrogen, carboxylic acid, and other elements found in banana peels have similar magnetic properties to heavy metals. The health of 12/42 people is not impacted by these chemicals. This is excellent news, as heavy metal poisoning of water is one of the major issues in water scarcity. Lead, copper, mercury, and iron are a few of the metals that can be discovered in untreated water. These are lethal to humans, building up slowly in our bodies and eventually leading to brain and nervous system damage. Heavy metals in water are generally positively charged, and the carboxylic acid ions in the banana peels become negatively charged. The two compounds are drawn towards each other just like magnets. The researchers found that minced banana peel could quickly remove lead and copper from river water as well as, or better than, many other materials. A purification apparatus made of banana peels can be used up to 11 times without losing its metal-binding properties, researchers note. The research team adds that banana peels are very attractive as water purifiers because of their low cost and because they don't have to be chemically modified in order to work.
Figure 2.2: Dry Banana Peel.
2.2 Fine Aggregate
Fine aggregates contain crushed stones with pure granular size, which is a basic ingredient in concrete. The granular size of fine sand is so fine that it can pass by a 4.75mm sieve. The fine aggregates are mostly used to fill the voids present between the coarse particles. They also help in producing workability in the mixture. Sand is a granular material composed of finely divided rock and mineral particles. Sand shall be clean and free from organic impurities.
Figure 2.2: Fine Aggregate.
Test for Fine Aggregate
Specific gravity test
Weight of the empty Pycnometer, WI = 597g Weight of the Pycnometer + Soil, W2 = 1425g Weight of the Pycnometer + Soil + Water = 1983g
Weight of the Pycnometer + Water = 1499g
Specific Gravity = W2-W1/(W2-W1)-(W3- W4)
= 1425-597/(1425-597)-(1983-1499)
= 826/826-484
= 2.415
2.3 Coarse Aggregate
Crushed aggregate is produced by crushing quarry rock, boulders, cobbles, or large-size gravel. Recycled concrete is a viable source of aggregate and has been satisfactorily used in granular subbases, soil- cement, and in new concrete. After harvesting, aggregate is processed (crushed, screened, and washed) to obtain proper cleanliness and gradation. If necessary, a benefaction process such as jigging or heavy media separation can be used to upgrade the quality. Once processed, the aggregates are handled and stored to minimize segregation and degradation and to prevent contamination. Aggregates strongly influence concrete's freshly mixed and hardened properties, as well as influencing mixture proportions and the economy. Consequently, the selection of aggregates is an important process. Coarse aggregates refer to irregular and granular materials such as sand, gravel, and crushed stone. They are used for making concrete. In most cases, Coarse aggregates are naturally occurring and can be obtained by blasting quarries or crushing them by hand or with crushers. It is imperative to wash them before using them for producing concrete. Their angularity and strength affect the concrete in numerous ways. Needless to say, the selection of these aggregates is a very important process. The graded coarse aggregates are described by the normal size (i.e) 20mm, 12.5mm.
Figure 2.3: Coarse Aggregate.
2.4 Grey Water
Grey Water composition varies according to source. The first source is Grey Water from Bathrooms: water used in hand washing and bathing generates around 50-60% of total Grey Water and is considered to be the least contaminated type of greywater. Common chemical contaminants include soap, shampoo, hair dye, toothpaste, and cleaning products. The household's activities vary according to socio-economic status, cultural practices, cooking habits, and cleaning agents used, as well as 16/42. For example, a household that uses phosphate-free laundry detergent produces a Grey Water that is much lower in phosphate than one that does not. Grey Water usually contains surfactants (anionic, cationic and amphoteric) which come from shampoos and detergent formulations (Eriksson, et al. 2002). Personal care products are usually found in 10% of bathroom Grey Water, while detergents are the main constituents of laundry Grey Water. The characteristics of Grey Water depend on are quality of the water supply, lifestyle and water usage pattern, living standards, social and cultural habits (soaps, toothpaste, shampoos, and detergent), and quality of the household chemicals used. The chemical composition will vary significantly in terms of both place and time due to the variations in water consumption in relation to the discharged amounts of substances.
Figure 2.4: Grey Water.
Test for Grey Water
Turbidity:
Turbidity is the physical parameter of water quality and it refers to the cloudiness or dirtiness of water, which is caused by fine susper 18/42 particles like silt, clay, organic and inorganic matter, etc.
Total Suspended Solids:
The suspended particles are particles that may not pass through filter paper. TSS is also called conventional pollution.
Chemical Oxygen Demand:
The Chemical Oxygen Demand (COD) is the amount of oxygen consumed by organic matter in a solution. In this research work, the closed reflux/colorimetric method was used to determine the COD in domestic Grey Water before and after adsorbent. According to SEQS, the COD of effluent must be less than 150 mg/L.
Biochemical Oxygen Demand:
The Biochemical Oxygen Demand is the amount of oxygen required to microorganisms for the decomposition of organic matter present in wastewater.
2.5 Sensors
pH Sensor
A pH sensor is one of the most important tools for measuring pH, and it is commonly used in water quality monitoring. This type of sensor is capable of measuring alkalinity and acidity in water and other solutions. When used properly, pH sensors can ensure the safety and quality of products and processes that occur in wastewater or manufacturing plants. There are many different types of pH sensors you can get for your application during liquid measurement; these may include combination pH sensors, laboratory pH sensors, process pH sensors, and differential pH sensors.
Combination pH sensors use a reference electrode and a measurement electrode. The reference electrode is used to provide a stable signal, while the measurement electrode is designed to detect any changes that occur with the pH value.
Differential sensors consist of three different electrodes, the third of which is a metal ground electrode. These sensors are unique in that they prevent reference contamination.
Laboratory sensors can be made from a combination sensor contained in a plastic body and 12 mm glass. These sensors are designed for lighter applications, such as pool monitoring and environmental sampling.
Process pH sensors are made from combination sensors but are built into a large, durable body that contains process connections for continuous pH monitor.
Figure 2.6: pH Sensor.
Turbidity Sensor
The Turbidity sensor is used to measure the cloudiness or haziness of the water, which is due to the amount of particles in water invisible to human eyes. The sensor uses light to detect suspended particles in water by calibrating the light transmittance and scattering rate. It changes with the water quality of total suspended solids (TSS). The acceptable value measured for the human body is from 0 to 5 NTU. The number that this sensor measures can be controlled by filters in order to avoid health issues due to the high rate of the suspended solids (TSS) in water.
Figure 2.7: Turbidity Sensor.
Ultrasonic Water Level Sensor
The ultrasonic water level sensor is used to send pulses of sound waves to the water surface on the tank and receive them back in order to calculate the water level in the tank. This helps avoid overflow from the water inlet and helps to determine the water volume in the tank.
Figure 2.8: Ultrasonic Sensor.
Rasperry Pi Sensor
The Raspberry Pi is a very cheap computer that runs Linux, but it also provides a set of GPIO (general purpose input/output) pins, allowing you to control electronic components for physical computing and to explore the Internet of Things (IoT).
Figure 2.9: Rasperry Pi Sensor.
Chapter 3 - Methodology
3.1 General
In this chapter, we discuss about the methodology of the design of a Biochar filter and its use to monitor the quality of treated Grey Water using IoT.
3.2 Methodology
Chapter 4
4.1 General
We are going to use the efficient method of biochar in place of charcoal filters since the carbon content is sufficient. Water is passing through a biochar filter. Then water is taken into testing and its value is noted. In addition to that, we are going to use raspberry pi, pH sensors, turbidity sensors, and ultrasonic sensors to determine the outputs. Our goals, among others, are:
- To measure perilous quality metrics like physical, chemical and microbial properties.
- To find the deviations in measured metrics and give timely warnings in recognition threats or hazards.
- To provide real-time analysis of the sensor data and recommend appropriate corrective measures.
4.2 Preparation of Biochar
The biochar used in this study was produced from banana peel and prepared in the laboratory using a pyrolysis process. The peel was collected from a local juice bar and dried in the sun for days. After drying, it was crushed with a blender to prepare three different sizes of biochar. The first was coarse biochar (CBC) which is > 3mm. The second biochar size was medium biochar (MBC), with size of 1-3mm. The last particle size was < Imm and it is referred to as fine biochar (FBC). The three different sizes were then carbonized by an electric muffle furnace at a temperature of 450 degrees for 1.5 hours and cooled at room temperature. The prepared biochar was washed with distilled water to remove the very fine particles and oven-dried at a temperature of 100°C for 3 hours.
4.3 Design of Biochar
The following steps are involved in the design of the Biochar filter:
- The filtration system consists of three layers, which are: coarse aggregate, fine aggregate, and biochar (Banana peel). In the filtration system, coarse aggregate of 20mm are used as a bottom layer at a height of 2.5 cm.
- A coarse aggregate of 10mm is also used as a layer, placed at a height of 4 cm above the bottom layer in order to keep the sand layer lying above the coarse aggregate layer. Sand passing through a 4.75mm sieve is used as a layer at two places: one is below the biochar layer with a height of 3 cm, and another one is placed above the biochar layer with a height of 3cm. Below the biochar, the sand layer is used to avoid the mixing of carbon content in water and also to avoid the mixing of layers.
- The biochar of banana peel is used as a charcoal layer to remove the impurities in chemical content. The top most layer of the sand layer, which is above the charcoal layer, is used in order to remove the large size particles.
4.4 Testing of Biochar Filter
Alkalinity Test
Alkalinity is a measure of the ability of the water to resist changes in pH. This is also known as buffering. For the majority of applications, it is important to maintain a stable pH. Alkalinity is a measure of the capacity of a solution to neutralize a strong acid. We can calculate the amount of alkalinity in water by titrating the sample water with acid, using an indicator to determine when the sample water's pH has dropped to a certain level: the endpoint.
Suspended Solids Test
“Suspended solids” refers to small solid particles that remain in water as a colloid or due to the motion of the water. Suspended solids can be removed by sedimentation (if their size or density is comparatively large) or by filtration. Total Suspended Solids (TSS) is one of the methods defined in this analysis. There is no specific chemical formula for a total suspended solid. Quite simply put, TSS is anything that is captured by filtering the sample aliquot through a specific pore size filter. Suspended solids can range from particles of silt or sediment to pieces of plant material such as leaves or stems. Even insect larvae and eggs can fall in the general category of TSS. High amounts of TSS can lead to an esthetically displeasing appearance of a body of water. Either the color or overall turbidity of the water will be negatively impacted.
Hardness Test
Hardness is commonly measured by colorimetric titration with an EDTA solution. A titration involves adding an indicator and then titrant solution in small increments to a water sample until the sample changes color. You can titrate a sample for total hardness using a burette or use a water hardness test kit. You can also measure calcium hardness separately from magnesium hardness by adjusting the pH and using different indicators.
Total Dissolved Solids Test
TDS in water is due to the dissolved salts and minerals in water which are usually present in the form of ions; ex- sodium, potassium, carbonates, sulphates, etc. Sometimes these dissolved solids can be toxic and/or cause a formation of scales in pipes, and hence determination of the same is essential. TDS can be determined by two methods:
- Gravimetric analysis: This method is a laboratory method and is time consuming, but the results are accurate. Here, the water sample is prepared by filtering water by a 1.5-micron filter so as to separate suspended solids from the water.
- TDS meter: Dissolved solids are usually present in water in the form of ions, and ions conduct electricity. This principle is utilized in finding the TDS of water. TDS meter tip is dipped inside water which measures the amount of electricity getting conducted, and this electricity value is calibrated to TDS value in ppm or mg/L. This method is very quick to use and is widely popular. The results of this test are approximate because all the dissolved solids present in water are not present as ions.
Biological Oxygen Demand
Biochemical oxygen demand (BOD) measures the amount consumed by microorganisms in decomposing organ 24/42 stream water. BOD also measures the chemical oxidation of inorgter (the extraction of oxygen from water via chemical reaction). A test is used to measure the amount of oxygen consumed by these organisms during a specified period of time (usually 5 days at 20°C). The rate of oxygen consumption in a stream is affected by a number of variables: temperature, pH, the presence of certain kinds of microorganisms, and the type of organic and inorganic material in the water.
Chloride Test
If water containing chlorides is titrated with silver nitrate solution, chlorides are precipitated as white silver chloride. Potassium chromate is used as indicator, which supplies chromate ions. As the concentration of chloride ions approaches extinction, silver ion concentration increases to a level at which reddish brown precipitate of silver chromate is formed indicating the end point.
Sulphate Test
A simple and precise turbidimetric method of determining sulfate S in water samples is described. It involves measurement of the turbidity formed when an aliquot of a barium chloride-gelatin reagent is added to an acidified sample. The method is sensitive and accurate and permits determination of microgram amounts of sulfate S present in water samples.
pH Test
The pH Testing Procedure is as follows: Rinse each test tube with the water sample. Gloves should be worn to avoid skin contact with the water. Fill the tube to the 5mL line with sample water. While holding a dropper bottle vertically, add 10 drops of Wide Range Indicator Solution. Cap and invert several times to mix. Insert the tube into the Wide Range pH comparator. Hold the comparator up to a light source. Match the sample color to a color standard. Record the pH value.
Chapter 5 - Testing of Filter and Conclusion
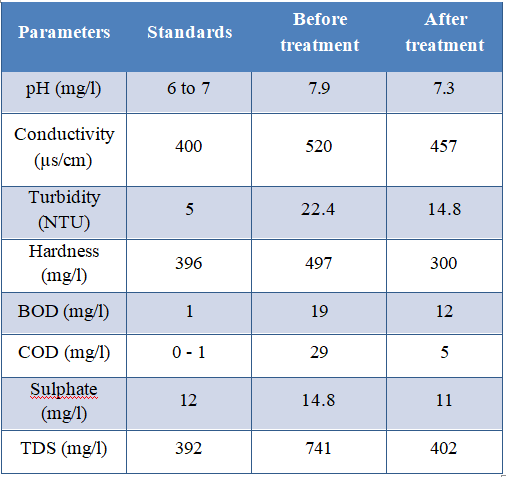
5.1 Test Result on Grey Matter After Treatment
5.2 Comparison of Grey Water Characteristics Before & After Treatment
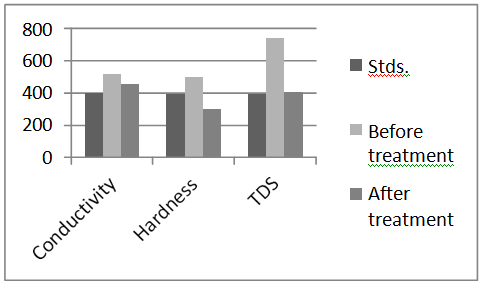
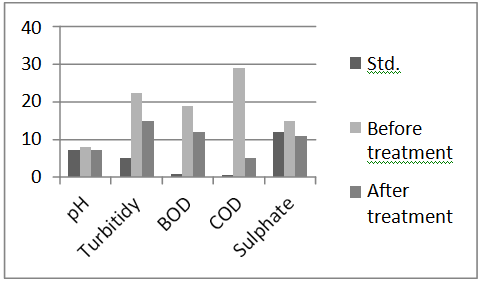
Below, chart 1&2 show variations of parameters of Grey water before and after treatment.
Chart 1.
Chart 2.
Conclusion
In the future, we will utilize biochar of another organic waste or agricultural waste in place of charcoal.
In this study, we tested only Grey Water with biochar. In future research, we are going to treat other types of waste water. For quick quality checking, we will implement the use of IoT based sensors to monitor the quality of water. pH sensors, turbidity sensors, and conductivity sensors are used to check the quality and determine whether a water sample is potable or not.
Here, we conclude that a biochar filtration system is an effective method for treating Grey Water. It yields better results when compared to normal filtration, which consists of charcoal and wood. Wood is a non–renewable resource; we must move on and turn to renewable resources as well as waste material. Large amounts of impurities are successfully removed using this biochar filtration.
This project also helps in solid waste management, as it helps to reduce solid waste and utilizes existing waste. The resultant water can be used for industrial, commercial, and irrigation purposes. Fresh water extraction is also reduced.
References
- Shaikh, Sk Sameer and Sk Younus, "Grey Water Reuse: A Sustainable Solution of Water Crisis in Pusad City in Maharashtra, India", International Journal on Recent and Innovation Trends in Computing and Communication, Vol 3:2, February 2015, pp.167-170.
- Manual on water supply and treatment. Ministry of Urban Development, New Delhi, 1999.
- Comparative test on household detergent powders, Consumer Voice, 2015
- Fangyue Li, Knut Wichmann and Ralf Otterpohl, "Review of the technological approaches for grey water treatment and reuses". Science of the total environment, Vol 407, February 2009, pp. 3439-3449.
- S Simon Jaboring. "Overview and feasibility of advanced grey water treatment systems for single households", Urban water Journal, 115. May 2013
- Guidelines for safe use of waste water, excreta and greywater - Excreta and greywater use in agriculture, World Health Organistaion,2006.
- Water reuse guidance manual factsheet, USEPA, 2012.
- Waste Water Engineering. Treatment and Reuse, Metcalf and Eddy. Fourth Edition.
- Almoayied Assayed, Jonathan Chenoweth and Steven Pedley," Drawer compacted sand filter. A new and innovative method for on- site gre water tractment", Environmental Technology. Vol 35:19. April 2014, pp. 2435 -2446.
- Ken Ushijima, Erina Tanaka, Lais Yuko Suzuki, Nowaki Hijikata and Naoyuki Funamizu Ryusei Ito, "Grey water treatment by slanted soil system with unsorted soil media", Environmental Technology, vol 36:20, May 2015, pp. 2603-2609.
- Ahmet Baban, Selda Murat Hocaoglu, Kemel Gunes, Selma Ayaz and Martin Regelsberger, "Grey water treatment and reuse by using RBC: A kinetic approach", Desalination and Water Treatment, Vol 23:1 - 3, August 2012, pp. 89-94.
- Nir Liberman, Semion Shandalov, Chaim Forgacs, Gideon Oron and Asher Brenner, "Use of MBR to sustain active biomass for treatment of low organic grey water", Clean TechnEnviron Policy. February 2016, pp. 1219-1224.
- J Gabarro, L. Batchelli, MD Balaguer, S Puig and J. Colprim, "Grey water treatment at a sports centre for reuse in irrigation: A case study", Environmental Technology. Vol 34:11, December 2012, pp.1385-1392.
- Boukary Sawadogo, Mariam Sou, Nowaki Hijikata, Drissa Sangare, Amadou Hama Maiga and Naoyuki Funamizu, Effect of detergents from grey water on irrigated plants: A case study", Journal of arid land studies. Vol 24:1, January 2014, pp. 117-120.
- Khalid Bani-Melhem and Edward Smith, "Grey water treatment by a continous process of an electrocoagulation unit and a submerged membrane bioreactor system", Chemical Engineering Journal, Vol 198- 199, May 2012, pp.210-210
To view all articles in this issue, please go to November 2023 eNewsletter. For a downloadable copy, please visit the IEEE Smart Cities Resource Center.




To have the eNewsletter delivered monthly to your inbox, join the IEEE Smart Cities Community.
Past Issues
To view archived articles, and issues, which deliver rich insight into the forces shaping the future of the smart cities. Older eNewsletter can be found here. To download full issues, visit the publications section of the IEEE Smart Cities Resource Center.